Passivation of stainless steel surfaces is essential for achieving long-term corrosion resistance and maintaining part reliability. This controlled chemical process restores the alloy’s natural protection after machining or welding. During fabrication and cleaning, residual free iron or contaminants can damage the oxide layer, leading to rust or pitting.
This guide explains what stainless steel passivation is, why it is needed, how the process works, the main passivation methods and standards, verification and safety practices, and answers to common engineering questions.
What Is Stainless Steel Passivation
The passivation process for stainless steel is a controlled chemical treatment that removes surface free iron and contaminants while promoting the formation of a dense, stable chromium oxide layer that greatly improves corrosion resistance.
Unlike coating or plating, stainless steel passivation does not add any material layer — it restores the metal’s own passive state and has almost no effect on dimensions or mechanical properties.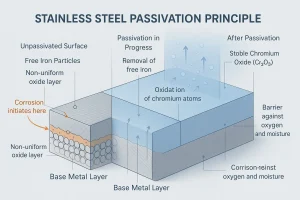
A Brief History of Stainless Steel Passivation
The concept of passivation was first recognized in the early 20th century when engineers observed that stainless steel naturally forms a thin chromium oxide film that prevents rust. In 1913, metallurgist Harry Brearley’s discovery of stainless steel confirmed this self-protective property.
By the 1930s, chemical treatments using nitric acid were introduced to restore and enhance this passive layer after machining or welding. These methods later evolved into modern standards such as ASTM A967 and AMS 2700, which continue to define today’s stainless steel passivation practices.
Why Perform Stainless Steel Passivation
Passivation treatment is widely used in precision manufacturing to ensure reliable corrosion performance and extended service life.
-
Improved corrosion resistance: Forms a dense chromium oxide layer that resists moisture, salts, and common chemicals.
-
Removes manufacturing residues: Eliminates free iron, polishing compounds, and oxides that could initiate rust.
-
Better cleanliness and hygiene: Produces a cleaner, more uniform surface suited for food, pharmaceutical, and medical uses.
-
Reduced maintenance cost: Minimizes downtime and replacements, improving overall life-cycle economy.
-
Weld repair: Removes discoloration and restores a consistent appearance in heat-affected zones.
Effective stainless steel passivation prevents premature corrosion and ensures consistent surface quality.
How Is Stainless Steel Passivation Performed
The stainless steel passivation process generally includes cleaning and degreasing → acid pickling/passivation reaction → multiple rinsing → drying and oxide-film regeneration or inspection.
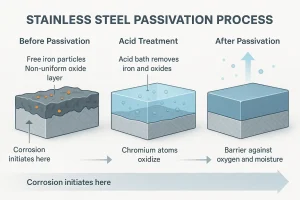
Each step directly affects the passivation film’s quality, consistency, and long-term corrosion resistance.
1. Cleaning and Degreasing
Use alkaline or neutral detergents at 40–60 °C for 5–15 min to remove oil, grease, and coolant residues from machining or polishing.
After rinsing, the surface should pass the water-break test — a continuous water film indicates full cleanliness, while droplets signal oil or surfactant residue.
Inadequate cleaning may cause dark spots or non-uniform oxide film formation later in the passivation process.
2. Acid Pickling and Passivation
Immerse parts in nitric (20–50 %) or citric (4–10 %) acid solutions to dissolve surface iron and promote chromium enrichment.
Nitric acid provides strong activation but requires fume extraction and waste neutralization; citric acid is safer and ideal for 304 / 316 grades.
Time and temperature must be carefully controlled — under-treatment leaves free iron, while over-treatment can dull the surface or cause pitting.
Typical parameters: 20–50 °C for nitric systems (20–30 min) and 50–65 °C for citric systems (10–40 min).
3. Multi-Stage Rinsing
Rinse parts three or four times using deionized water to remove residual acids and salts.
High-purity components often require a final hot DI rinse (60–80 °C) to prevent chloride contamination.
Conductivity should remain below 50 µS/cm, confirming that all active ions have been removed before drying.
4. Drying and Film Formation
Dry components with filtered hot air or in a clean oven below 80 °C to avoid oxidation marks.
During drying, the chromium oxide layer naturally reforms in contact with oxygen — within several minutes, the protective passive film stabilizes and provides full corrosion resistance.
Inspection should confirm a uniform, stain-free surface ready for testing or assembly.
Common Passivation Methods
Different passivation methods are available for stainless steel surfaces. Each offers varying levels of effectiveness, environmental impact, and suitability for specific grades or industries.
| Method | Chemical System | Key Features and Applications |
|---|---|---|
| Nitric Acid Passivation | HNO₃ (20–50 %) | Traditional, strong iron removal; requires fume control and neutralization. |
| Citric Acid Passivation | C₆H₈O₇ (4–10 %) | Safer and eco-friendly; ideal for 304/316 and hygienic industries. |
| Nitric + Sodium Dichromate | HNO₃ + Na₂Cr₂O₇ | High intensity but contains Cr⁶⁺; being phased out due to toxicity. |
| Electropolishing (with Passivation) | H₃PO₄ + H₂SO₄ mixtures | Removes micro-peaks and forms a superior oxide layer; used for mirror or high-purity parts. |
The choice of passivation method depends on alloy grade, surface finish, and environmental regulations in line with standards such as ASTM A967 or AMS 2700.
Main Benefits of Passivation

Key benefits of stainless steel passivation include improved corrosion performance and extended service life:
-
Enhanced corrosion resistance against pitting and crevice attack.
-
Restored intrinsic performance by removing embedded particles and free iron.
-
Longer service life and lower cost through reduced maintenance and downtime.
-
Improved surface appearance and cleanliness, easier to sanitize.
-
Weld zone restoration, eliminating heat tint and discoloration.
Passivation Standards
International passivation standards define chemical solutions, process parameters, and verification methods to ensure consistent corrosion resistance in stainless steel components.
| Standard | Scope and Description | Typical Applications |
|---|---|---|
| ASTM A967 | Defines nitric and citric acid concentrations, temperature, duration, and acceptance tests; supersedes QQ-P-35. | General machined parts, fasteners |
| AMS 2700 | Aerospace specification emphasizing chemical control and corrosion testing. | Aerospace and defense components |
| ASTM A380 | General guide for cleaning, descaling, and passivation of stainless steels. | Industrial equipment and piping |
| ASTM B912 | Recognizes electropolishing as an effective passivation process. | Medical, semiconductor, sanitary parts |
Verification and Testing
Passivation quality verification ensures that the oxide layer is fully developed and corrosion-resistant.
Common validation procedures include:
-
Water-break test – Observe whether water forms a continuous film; breaks or droplets indicate contamination or incomplete film.
-
Copper sulfate test – Immerse in CuSO₄ solution; copper deposition indicates free iron and insufficient passivation.
-
Salt-spray test – Expose to controlled salt fog to evaluate protective performance; duration depends on standard.
-
Humidity or immersion test – Simulate condensation or long-term exposure; check for rust, stains, or discoloration.
If the test fails, common causes include inadequate pre-cleaning, uneven acid treatment, poor rinsing, or incomplete drying.
If no stainless steel passivation is performed, residual free iron oxidizes quickly in humid or chloride-rich environments, creating rust spots and shortening service life.
Safety Precautions
Safety in stainless steel passivation operations is crucial for both workers and equipment longevity.
-
Personal protection: Use acid-resistant gloves, goggles or face shields, and aprons.
-
Ventilation: Operate nitric systems with effective fume extraction.
-
Chemical control: Maintain proper concentration, temperature, and duration to prevent over-etching or discoloration.
-
Avoid mixed materials: Do not process different stainless grades in the same bath.
-
Thorough rinsing: Multi-stage DI-water rinse prevents acid or chloride residue.
-
Waste handling: Neutralize and dispose of waste solutions according to regulations.
Strict adherence to safety guidelines ensures stable and repeatable passivation results.
Frequently Asked Questions
Q1 Is passivation always required for stainless steel?
It is strongly recommended for food, medical, marine, and chloride environments; optional for mild indoor conditions.
Q2 Which acid should I choose — citric or nitric?
Citric acid is safer and eco-friendly; nitric acid removes iron more aggressively but requires tighter waste control.
Q3 Does passivation change dimensions or color?
It causes virtually no dimensional change; surfaces appear slightly brighter and cleaner.
Q4 How long does the passivation film last?
It remains stable under normal use; periodic inspection is advised for parts in chloride or high-humidity environments.
Q5 How does passivation differ from electropolishing?
Passivation chemically removes free iron and reforms the oxide layer, while electropolishing smooths the surface and simultaneously creates a higher-grade passive film.
Conclusion
Proper stainless steel passivation significantly improves corrosion resistance, appearance, and cleanliness while reducing total life-cycle cost.
Following ASTM A967 or AMS 2700 and performing water-break, copper-sulfate, or salt-spray tests ensures consistent, repeatable quality.
Ready to start your passivation project? Upload your drawings and our engineering team will provide an optimized passivation process and quotation.



