Electropolishing is an advanced metal finishing technique that plays a crucial role in modern precision manufacturing.
By removing a controlled layer of material through an electrochemical process, engineers can achieve a clean, smooth, and highly corrosion-resistant surface that meets the demands of critical industries.
The following sections explain what electropolishing is, how it works, which materials it suits best, when to use it, and why it remains one of the most effective finishing methods for high-precision components.
What Is Electropolishing
Electropolishing is a precision surface finishing process that removes microscopic roughness from metal parts using electrochemical dissolution.
The part is made the anode in a direct-current electrolytic cell, and a controlled voltage causes metal ions to dissolve preferentially from surface peaks, leaving a bright and smooth finish.
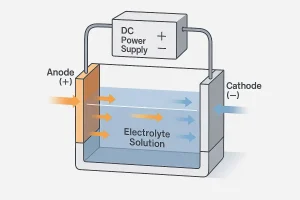
Illustration of the electropolishing process showing how current flows from the anode to the cathode through the electrolyte solution.
Unlike mechanical polishing, electropolishing does not rely on abrasive contact, which means it preserves dimensional accuracy while improving corrosion resistance and reflectivity.
It is commonly used on stainless steel, titanium, and nickel alloys in applications that require excellent cleanliness and aesthetics.
How the Electropolishing Process Works
Electropolishing functions within an electrolytic bath where the workpiece acts as the anode (+) and a stainless steel or lead plate serves as the cathode (–).
Both electrodes are immersed in a mixture of phosphoric and sulfuric acid.
When direct current passes through the bath, high points on the metal surface dissolve faster than low points, resulting in a leveled, mirror-like finish and a stable passive layer.
Process Steps
- Pre-cleaning – Remove oils, oxides, and surface contaminants.
- Electropolishing – Submerge parts in the electrolyte at 50–80 °C under 10–30 A/dm² current density.
- Rinsing and Neutralizing – Eliminate any residual electrolyte and dissolved ions.
- Drying and Inspection – Verify the uniformity of brightness and finish.
Materials Suitable for Electropolishing
Electropolishing can be applied to most conductive metals, though stainless steel remains the most widely used.
| Material | Recommended | Description |
|---|---|---|
| 304 / 316L Stainless Steel | Yes | Produces bright, passivated surfaces with Ra reduced to 0.1 µm. |
| Titanium and Titanium Alloys | Yes | Suitable for medical and aerospace components requiring biocompatibility. |
| Copper and Brass | Limited | Requires modified electrolytes to prevent over-dissolution. |
| Aluminum / Magnesium | No | Highly reactive and unsuitable for common acid baths. |
When to Use Electropolishing
Electropolishing is preferred when components must achieve a highly smooth, clean, and corrosion-resistant finish, particularly when mechanical polishing is infeasible or could affect tolerance.
Typical situations include:
- Removing micro-burrs and tool marks after CNC machining.
- Enhancing corrosion resistance for stainless steel and titanium parts.
- Achieving a bright, reflective finish without mechanical buffing.
- Finishing complex internal geometries or hollow parts.
- Pre-treating surfaces before passivation, coating, or vacuum sealing.
Advantages of Electropolishing
- Achieves superior surface smoothness with Ra 0.05–0.2 µm.
- Improves corrosion resistance by forming a stable passive oxide layer.
- Removes sharp edges and fine burrs without distorting the part.
- Reduces residual stress and surface contamination.
- Ensures high cleanliness for medical, food, or vacuum-grade parts.
Limitations
- Applicable only to conductive materials.
- Requires controlled handling due to acidic electrolytes.
- Less effective in deep holes or enclosed cavities.
- Processing costs are higher than for basic mechanical polishing.
Comparison with Passivation
Although both electropolishing and passivation improve corrosion resistance, they differ in mechanism and results.
| Aspect | Electropolishing | Passivation |
|---|---|---|
| Principle | Removes surface peaks via anodic dissolution. | Forms a thin oxide film by chemical reaction. |
| Surface Appearance | Bright and reflective. | Matte gray. |
| Material Scope | Stainless, titanium, nickel alloys. | Primarily stainless steel. |
| Material Removal | 5–40 µm controlled removal. | No material removal. |
| Cost | Slightly higher. | Lower. |
Electropolishing should be chosen when high reflectivity and ultra-smooth surfaces are required, while passivation suits general anti-corrosion protection.
Applications of Electropolishing
- Medical and Pharmaceutical Equipment: Achieves sterile, biocompatible surfaces.
- Aerospace Components: Improves fatigue strength and sealing performance.
- Food and Beverage Machinery: Meets sanitary-grade surface standards (Ra ≤ 0.4 µm).
- Vacuum and Optical Components: Reduces outgassing and contamination.
- Semiconductor Equipment: Provides ultra-clean finishes for high-purity environments.
Conclusion
Electropolishing delivers precise surface enhancement that combines smoothness, corrosion resistance, and cleanliness.
It supports industries that demand flawless surface quality, from aerospace and medical devices to semiconductor manufacturing.
Request a quote from our engineering team to discover how electropolishing can improve the performance and appearance of your next project.